Relaxing Dishes
For wetting specimens, a container is used which is equipped with a layer at the bottom to maintain a moist environment (paper towels, washed river sand). This can be a Petri dish or a plastic or glass low container. Moisten the bottom of the container (dropper, syringe), add an anti-mould substance (10% vinegar, Ajatin, Thymol) and carefully place the specimens to moisten. Place specimens in paper bags with the bags to minimize the risk of damage during handling. Uncap specimens in tubes and place on the bottom of the dish. After approximately 10 to 15 hours, the specimens can be transferred from the bags or tubes to the bottom of the dish, a little water added and the softening process continued.
Dropper / Wash bottle
Anti-mould agent
Cleaning brushes
It is advisable to subject dampened specimens to at least a basic cleaning process to remove dust and common dirt before actual preparation. In the case of more soiled specimens, intensify the cleaning process by using a suitable solution - water, soapy water, solvent.
Depending on the nature of the specimen to be cleaned, we use brushes from size 0 to 12.
Chemical cleaning and degreasing
To increase the cleaning effect and for degreasing we usually use the following solvents:
- ethyl acetate (cleaning, degreasing, mould)
- benzene (cleaning, degreasing)
- ether (cleaning, degreasing)
- alcohol (cleaning, degreasing)
- chloroform (mould)
- hexane (cleaning, degreasing)
Preparation pad
A plate of 3 cm high polystyrene or plastazol serves well as a preparation pad. In the case of polystyrene, it is advisable to cover the plate with filter paper, which absorbs body fluids released during drying of larger specimens. It is advisable to have more than one of these plates ready, as the prepared material is left to dry on them.
Tweezers
Preparation needles
Preparation needles are used to flatten limbs and antennae during preparation. We use needles that are straight or slightly curved. Depending on the size of the material to be prepared, the needles may also have different diameters. In the beginning we make do with an entomological pin, but with increasing practice everyone will find the needle (shape and thickness) that suits them best during preparation. Then we buy professional needles or we can make such needles from entomological pins of suitable thickness, which we glue into a suitable handle. The handle is made from a wooden log or an ordinary pencil, into which a hole is drilled, and a suitable entomological pin is fixed into the hole using instant glue.
Preparation brush
Specimen fixation cover slip
When actually preparing small specimens, it is advisable to hold the specimen firmly yet gently. For this purpose, a preparation made of a microscopic cover slip is used, which is fixed on a small piece of cork, polystyrene or plastazol.
The microscopic glass slide is soft, flexible and strong enough - just enough to keep the specimen from being crushed and to hold it securely in place while we groom the antennae and limbs.
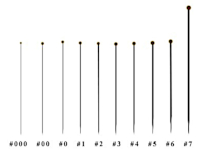
Entomological pins
These are special pins made of steel wire with a basic length of 38-39 mm, which are varnished to prevent rusting from the body fluids of the prepped specimens. They are manufactured in thicknesses of 000 to 7 (see table).
| Usual parameters of entomological pins | ||
| Pin size | Diameter [mm] | Length [mm] |
| 000 | 0,25 | 38 - 39 |
| 00 | 0,30 | 38 - 39 |
| 0 | 0,35 | 38 - 39 |
| 1 | 0,40 | 38 - 39 |
| 2 | 0,45 | 38 - 39 |
| 3 | 0,50 | 38 - 39 |
| 4 | 0,55 | 38 - 39 |
| 5 | 0,60 | 38 - 39 |
| 6 | 0,65 | 38 - 39 |
| 6A | 0,60 - 0,65 | 45 |
| 7 | 0,60 - 0,70 | 52 |
When preparing Czech beetles, we use pins of size 1 - 4, the most used being size 2 and 3. We use size 1 pins exceptionally for poorly sclerotized or very narrow individuals and size 4 pins for the largest individuals (approx. > 35 mm).
Fixation pins
We use these pins to fix the impaled specimen. We use them to anchor not only the body of the specimen to be prepared, but also to fix the flattened limbs and other growths (antennae, tentacles) on the preparation plate. For this purpose, we can also use ordinary tailor's pins or entomological pins.
I use tailor's pins for larger specimens (approx. > 20 mm), for smaller specimens (15 - 20 mm) I have reserved stainless steel pins number 000.
Glue
The glue is used to attach the prepared specimen to the sticker. The following requirements are placed on a quality entomological glue:
- it must be non-toxic
- it must have a high affinity for the chitin body of the beetles
- it must be water-soluble
- it must be transparent
- it must be flexible
The above requirements are met without fail by Herkules glue, which has been the most used glue by Czech entomologists for many years. Other types of glues are practically not used by Czech amateur entomologists. In entomological supplies you can buy glue based on glue (approx. 200.- CZK/35 g) or glue based on polyvinyl alcohol (approx. 120.- CZK/30 g).
Mounting boards
These mounting boards are most often made of white cardboard and come in a wide range of sizes and shapes. There are also transparent labels, but these are not widely used.
The mounting boards are most often rectangular in shape, but there are also pentagonal and triangular labels. On these labels, specimens are glued crosswise, head to left.
Over time, each entomologist chooses a set of mounting boards that best suit his or her size and uses these as standard. The size of the labels should be chosen so that the label fits the specimen, does not extend beyond the edges of the label, but is not "drowned" on the label, i.e. the label is not disproportionately large in relation to the size of the specimen.
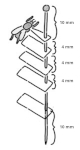
Pinning block
This tool ensures that the actual specimens, all descriptive tickets (locality, determination, supplementary), and possibly other parts of the specimen (excised copulatory organs) are placed at the same height. This helps to maintain the aesthetic level of the collection.
The standard length of an entomological pin is 38 mm. In order to avoid damage to the specimen when handling the pin, it is necessary to keep a distance of about 10 mm between the head of the pin and the specimen itself. For this reason, large specimens are impaled at a height of 10 mm from the top edge and small specimens are impaled at a height of 25 mm from the bottom edge of the pin.
The locality labels shall be placed at a height of 21 mm from the bottom edge of the pin, If additional labels with supplementary locality data are used, they shall be placed below the main locality label.
Determination labels are placed below the locality labels (main or supplementary) and can be placed e.g. at a height of 10 mm, i.e. at the bottom of the entomological box.
The standard heights that each label should contain are therefore 10, 21 and 25 mm. If we decide to use labels with additional locality data (which is not very common), we should have a height label that includes other possible positions (e.g. 17 and 13 mm).
Magnifier / Microscope
A good preparation loupe is essential when preparing specimens, especially smaller ones. The so-called spectacle magnifiers are very useful in this respect. These are magnifiers that the preparator wears over his eyes like glasses. This leaves both hands free for handling the specimen being prepared. For the purpose of preparation, I recommend choosing a magnifying glass with a magnification of 10x - 15x and preferably with the possibility of choosing both of these magnifications. I have had good experience with a magnifying glass that allows the choice of 10x - 15x - 20x - 25x magnification. I mainly use the 10x and 15x magnification for my own preparation. The other two magnifications can be used only to a limited extent during the preparation, because there is a very small distance between the examined object and the magnifying glass, which does not allow comfortable use of the preparation tools (preparation needle, preparation brush, cover glass to hold the specimen). However, I use the higher magnification e.g. for determination.
A good binocular microscope is suitable for preparation of the smallest specimens. There are a number of microscopes on the market in different price ranges. It is an instrument that is more expensive to acquire. A beginner entomologist can get by with a good magnifying glass at first, and I recommend buying a microscope only after gaining more experience, with the proviso that you need to think carefully about which one to get. The purchase price of a good binocular microscope starts at about 15 000 CZK. When choosing one, I recommend looking at the following parameters:
- the overall rigidity of the construction (the cheapest microscopes may blur due to their unstable construction)
- basic optical magnification (for preparation, a magnification of up to 45x is sufficient, for the use of the microscope and for determinations I recommend choosing a maximum magnification of at least 90x)
- the choice of magnification (fixed vs. zoom: each has its advantages and disadvantages, I prefer zoom for convenience)
- min. distance of the lens from the object to be observed (this determines the possible working space during preparation)
- the possibility of buying accessories (eyepieces, lenses, filters, eyepieces)
- possibilities of taking a photographic record (e.g. third tube)
Location labels
I consider the marking of each specimen with a locality label to be as important as the preparation itself. A well-prepared specimen without a good quality locality label is of no value to the collection.
The locality label is placed under the prepared specimen. For beetles glued to labels, it is placed at a height of 21 mm from the tip of the pin. Beetles impaled on a pin are usually of greater thickness and the locality label is thus placed several mm below the impaled specimen.
Locality tags carry information about the location of the find, the date of the find and the collector who found the beetle. It is a good idea to follow certain rules when writing locality tags. Keep in mind that the contents of the labels should be clear even to entomologists who will study the record in perhaps 100 years.
There is no standard regarding the size of locality labels. However, the unwritten rule is that the locality label should not be much larger than the adhesive label with the specimen itself. In the literature you will usually find a recommendation that the maximum size for a locality label is 7 x 18 mm. Today, locality labels are most commonly printed on printers. The recommended font size is 3pt and a legible, preferably sans serif font such as Calibri or Verdana in Windows or Ubuntu, Liberation Sans or Dejavu Sans in Linux.
Rules for writing location labels:
Locality
Always indicate the country in which the specimen was found. For the Czech Republic, it is customary to use Bohemia and Moravia. It is also possible to use the international designation CZ.
In addition, give a more detailed indication of the place of discovery. The name of the municipality is given according to the cadastral area, which can be found e.g. on mapy.com. Avoid using local or unofficial names.
At present, I highly recommend adding GPS coordinates to the location. Use the WGS84 format to indicate 50.3059672N, 14.4967869E or 50°18'22 "N, 14°29'48 "E. You can also use the shorter notation 50.3059672 14.4967869
The usual abbreviations used on location tickets for a site:
- cent. = centralis - central;
- sept. = septentrionalis - north;
- bor. = borealis - northern;
- mer. = meridionalis - southern;
- or. = orientalis - eastern;
- occ. = occidentalis - western;
Date
Always indicate the date of the find by writing the month number in Roman numerals and placing it between the day and year of the find, e.g. 26.vii.2025 or 3.xi.2019 or 26-vii-2025.
Collector
The information about the finder of the specimen is written in the form of the lgt. name, e.g. Petr Dudek lgt. or P. Dudek lgt.
The abbreviation that follows the name is either lgt. or leg. and means legit = collected.
Additional locality labels
Some collectors and institutions place one or more additional labels under the main locality label, giving further details of the find such as altitude, food plant or parts of it, type of trap used, a more detailed description of the locality, and the registration number in the database or journal.
Scalpel / Surgical scissors
The following two tools can be used when preparing Meloe adults or larvae. For more detailed descriptions of each, see the "Preparation methods" page.
Backing and cover glasses
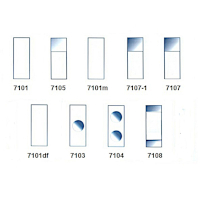
When preparing microscopic slides, the prepared objects are usually placed on standard slides, which have a standard size of 26 x 76 x 1.1 mm. There are also a number of special slides which may be of a different size or contain one or two wells, or have a part of the surface etched to describe the slide.
The slide is then covered with a cover glass, which can also be of different size and shape (square, rectangle, circle).
Personally, I use the basic type 7101 slides and cover glasses of 15x15 mm, 18x18 mm or 22x22 mm.
Phibre rings
Phibre rings are used in the preparation of slides in which the object to be prepared is enclosed in a special chamber consisting of a slide, a cover glass and a phibre ring. This chamber may be filled with air or with a special sealing medium.
I create such microscopic specimens with the chamber filled with air in selected smallest specimens (< 3 mm).
Personally, I use 1 or 1.5 mm thick fibre rings in sizes 12x16 mm, 14x18 mm and 16x20 mm. The first digit indicates the inner diameter, the second digit the outer diameter.
Solacryl BMX
Solacryl BMX is a solution acrylic resin based on a copolymer of butyl methacrylate and methyl methacrylate dissolved in xylene.
It is mainly used for wood restoration and preservation, coating concrete floors and interlocking paving, and preparing microscopic specimens. It is a viscous liquid with the smell of xylene, in which the resin is dissolved. The density of the solution is 0.9 g/cm³. The resin is insoluble in water and dissolves in benzene, toluene, xylene, acetone, or ethyl acetate.