Collecting insects, identifying them and compiling collections is not in itself a scientific activity, but any purposeful collection can be used for such scientific activities. Biological collections are of immense value as archives of biodiversity and as scientific resources for taxonomic and applied research. Collections can fulfil a number of important functions:
- they allow the safe and rapid identification of insects, which is a prerequisite for any biological study,
- provide material for the study of systematic problems, and give an overview of geographical and individual variation within species,
- provide material for comparative morphological studies,
- they preserve type specimens with an important nomenclatural function,
- provide a wealth of information on the ecology and phenology of individual species,
- they are the basic source of material for most zoogeographical and faunistic studies.
The material for the collection is mainly obtained by our own collection, but there are other ways to obtain material for the collection:
- Exchange between collectors
This is the traditional method by which entomologists exchange duplicates from their collections with each other. There are also specialised exchange lists (e.g. Entomology Forum) or you can contact colleagues in the field (e.g. regional entomologists, museums, universities). When exchanging, ALWAYS follow the ethical rule and provide accurate information about the locality, date and collector. - Purchase or auction
There are specialist shops on the internet selling dried entomological material or running auctions of entomological material (e.g. eBay, Delcampe, insectnet.com, insect-trade.eu, insect-classifieds.com). When buying, make sure that the specimens are of legal origin - some species are protected by CITES or national legislation. You can also acquire new specimens for your collection by buying them at one of the many organised exchanges (see the Events menu). - Material from museum or university sources
Many museums and universities allow you to study or borrow material (especially for research purposes). In some cases it is possible to obtain duplicates from determinate collections which are released for exchange. It is thus advisable to make professional contact with the relevant curator of the entomological collection. - Cooperation with field entomologists
You can participate in joint expeditions or field research where you have the opportunity to obtain some of the material. For example, in biological inventories or monitoring projects (nature conservation, ecological studies). - Breeding and rearing
Some species of beetles can be reared from larvae or pupae, e.g. from dead wood, dung or fungi. This method is ethical and allows observation of the developmental stages. You can also contact breeders of exotic beetles (e.g. Dynastes, Lucanus, Goliathus, etc.).
When studying insects, we divide collections into the following basic groups:
- wet collections (collections of specimens stored in preservative liquid),
- dry collections (collections of dry specimens),
- microscope slide collections (collections of slides enclosed in a medium such as air, Canada balsam, ...),
- associated collections (this includes, for example, collections of genitalia, juvenile stages (e.g. larvae), photographic records, and the like).
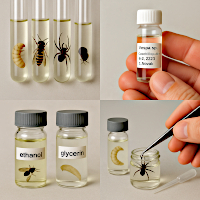
Wet collections
Preservation fluid storage is used for soft-bodied insects (e.g. larvae, pupae, some adults, arachnids) or where insects cannot be dried without deforming the body.
These are usually collections of specimens stored in ethanol or other preservative liquid (formaldehyde) in glass tubes. Fill the tube with the specimen, labelled with the locality and determination label, to the brim with preservative liquid, plug it with cellulose cotton wool and place it bottom up in the storage bottle. Fill the storage bottles in which the samples are kept with the same preservative liquid and close them with a tightly sealing lid. Keep samples belonging to a particular taxonomic group in the storage bottle. Label each storage bottle with a description.
The most commonly used preservative solutions are:
- For morphological studies, 70 - 80% ethanol (alcohol), which serves as a universal preservative (or 75 - 80% denatured ethanol).
- For molecular analyses (DNA), live samples are placed directly into 95-100% ethanol and kept as cold as possible (for long-term morphological storage, you can then convert to 70 - 80% ethanol). Avoid denatured alcohol and formaldehyde for DNA.
- Formaldehyde (4%) is less used, most often used only for short-term fixation.
- Glycerin solutions are used to maintain tissue elasticity and in genital preparation.
- As an emergency substitute, 70 - 85% isopropanol can be used, which works for morphology but is not ideal for DNA.
Store the test tube bottles in a cool, dark place if possible and check for evaporation of the preservative fluid at least once a year. Replenish the amount if necessary.
When building a beetle collection, we opt for a wet collection in the following cases:
- preservation of larvae and pupae (soft-bodied, quickly deformed),
- also suitable for very small/soft adults (e.g. parts of Staphylinidae) or for temporary storage or transport,
- in genital preparation, where the preparation is not glued to a label but kept in a microtube (microvial) with glycerine, pinned under the adult.
However, most adults are prepared "dry" as a standard. However, if it is necessary to preserve the adult in liquid (e.g. temporarily or very small pieces), expect that pigment colors may fade.
Dry collections
Place the recovered dry material on labels or pins labeled with the location of collection, date found, collector's name, and a determination label containing the genus and species name in entomological boxes.
In the first stage, we place the dry material in the so-called storage boxes. Once we have enough material in the stock boxes (usually after the season), we can proceed to reassign them to standard collection boxes. There we sort the specimens according to taxonomic units: orders, families, genera, species and subspecies. For more information on the organization of the collection, see the menu "Determination and organization of the collection".
The standardized entomological boxes, called "museum boxes", are made of cardboard with a bottom of plastazote, an insulating material 10 mm thick. In the past, polystyrene, porous, plastisol were used, and the great misfortune for the collection used to be compressed peat or cork (these natural materials were porous and became an ideal environment for the development of pests - the disturber, the worm-eater, the leatherjackets). The box is covered with a solid or glazed lid and covered usually with black wallpaper or canvas. The dimensions are usually 23 × 30 cm, 30 × 40 cm and 40 × 50 cm. The boxes must be of good quality, i.e. they must be sufficiently tight to prevent pests from getting in and to prevent the substances used to protect the collection from leaking out.
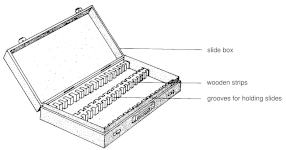
Collections of microscopic slides
Microscope slides are usually stored in wooden or plastic boxes. The inner sides of the boxes are slotted to allow the slides to be vertically mounted and separated from each other. Slide boxes are available in sizes designed for 50 to 100, sometimes more, slides.
For large slide collections, there are slide boxes or cabinets that contain a series of 'drawers' in which the slides are stored horizontally.
| Table: Methods of storing insect microscopic slides | ||||
|---|---|---|---|---|
| Type of slide | Use | Medium used | Suitable insect groups | Notes |
| Permanent microscopic slide (whole body) | Study of general morphology of small species | Canadian balsam, Euparal, Hoyer's solution | Aphids, lice, fleas, small parasitoids | Requires decolourisation in KOH and drying |
| Preparation of body parts (e.g. genitalia, wings) | Study of taxonomic characters | Glycerin, Euparal, polyvinyl lactate | Beetles, bipterans, passerines | Often deposited in microtubes attached to a pin |
| Wet preparations (in liquid) | Study of soft tissues, larval stages | 70 - 80 % ethanol, 5 % formalin, glycerine solutions | Mosquito larvae, flies, dragonflies | Must not be stored in light, tight caps required |
| Dry preparations (dried specimens) | Study of external morphology, identification | No medium (air-dried) | Beetles, butterflies, flatworms | Long-term stability, dryness and protection from pests required |
| Temporary aquatic preparations | Short-term observation of live or fresh specimens | Water or saline solution | Aquatic insect larvae | Perishable preparation, suitable for short-term use only |
In the case of beetle collections, one of the following types of microscope slides is encountered:
- a permanent microscopic slide of all or part of the body where the slide is embedded in a liquid medium such as glycerin, Canada balsam or Euparal,
- a dry microscopic slide of the whole body, where the slide is placed in an air chamber to protect it from damage.
Associated collections
This group contains mostly supplementary material and documents related to the main (dry) collection. Such supplementary material can be, for example, typical specimen specimens of individual species, herbarium with plants typical for individual species or, for example, photo documentation of typical habitats or developmental stages. A well kept field diary or notes on the rearing of individual species can be a supplementary document. A well-maintained database in electronic form can also be a document, although there is a question of the long-term (e.g. > 100 years) sustainability of such a database.