Collection preservation tools
Traditional insecticides such as Lindane (gamma-HCH) are no longer used in many countries, including the Czech Republic, for the protection of museum and entomological collections due to their high toxicity and environmental damage and are included on the list of banned substances (Stockholm Convention). Modern collection protection focuses more on non-chemical methods and more environmentally friendly chemical alternatives.
Modern methods and insecticides for entomological collections
The following approaches are used today to protect entomological collections:
- Physical and Preventive Methods (Preferred Methods)
These methods are the most gentle for exhibits and reduce the need for chemistry- Fumigation (nucleation): this is the most common and effective method. Infested boxes are placed in a freezer (usually at -20˚C or below) for several days to a week. This kills all developmental stages of the pest (eggs, larvae and adults).
- Tight storage: the use of tightly sealed entomological boxes is essential. This will physically prevent pests from entering.
- Microclimate control: Keeping the relative humidity low (ideally below 50%) prevents the development of mould and some pests (e.g. mites).
- Monitoring: Use of pheromone traps (e.g. for jammers or moths) to detect pests early.
- Chemical Alternatives (Backup and local interventions)
If chemical intervention is necessary, more benign substances are used- Pyrethroid fumigants: for rapid destruction in closed depositories (e.g. based on substances such as cyphenothrin, deltamethrin). They are effective on adult and crawling insects, but their use requires special procedures and evacuation of the premises.
- Phosphine fumigation (PH3): very effective and used in professional collections and archives. It is used by specialist companies in sealed chambers because it is poisonous. It works deep into materials and destroys all developmental stages.
- Aromatics: some collections still use substances such as Thymol, camphor or naphthalene (instead of the original Lindane), which have a repellent effect, but their effectiveness against leatherjackets larvae is often insufficient and they are also toxic.
Important note: Most commercially available insecticides are intended for plant protection, crop protection or household disinfestation (e.g. sprays for cockroaches, aphids, etc.). These products are not suitable for long-term storage in entomological boxes because they may react chemically with the formulations, paints or box material.
Therefore, for amateur collections, a combination method is the safest and most common:
- Tight boxes.
- Regular inspection.
- When pests are found, a quick and thorough ERASE.
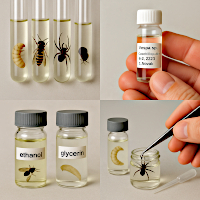
Containers for preserving wet collections
Containers for preserving wet collections are an essential means for long-term preservation of insects and other arthropods in preservative liquids. They are mainly used for soft-bodied specimens that cannot be prepared on pins (larvae, pupae, parasitoids, small insects, aquatic species, etc.).
Storage containers (also sometimes called preservation jars, wet archives or spirit collections) are used for:
- long-term storage of specimens in liquid (so-called 'wet specimens'),
- protecting biological material from drying, decomposition and mould,
- permanent archiving of soft or fragile insect body parts,
- clear record-keeping of collection specimens in museums and scientific institutions.
These containers form the "wet part of the entomological collection", complementing dry collections of impaled specimens.
They usually consist of
- The body of the container
Material: clear laboratory glass (most often borosilicate, e.g. Duran, Pyrex) or exceptionally clear plastic (PET, PP) for smaller volumes.
The most commonly encountered wide-mouth (for easy insertion and removal of slides) glasses are cylindrical, flat-bottomed and have volumes from 20 ml to 2 litres, depending on the type of specimen. - Cap
Proper capping is crucial for the long-term preservation of wet collections.
The cork is traditional, easy to fit, but dries out over time and leaks; it is therefore often coated with paraffin or wax. The rubber stopper, although a good seal, can react with alcohol, so it is recommended to coat it with paraffin as well.a plastic or Teflon plug (PTFE) is a modern option with high durability and chemical stability. A screw cap with a gasket (PE, rubber or Teflon liner) is now the most common for museum use systems. - Designation and description
Each container has:
- inner label made of paper written in ink or laser printed on alcohol resistant paper, dipped in preservative liquid,
- an outer label on the body of the jar, with inventory number, type, location and other information,
- in the case of large collections, a barcode or QR code for digitisation.
the most common use in practice:
- Preservation of soft-bodied developmental stages
Larvae, pupae, caterpillars, aquatic species (e.g. Chironomidae, Ephemeroptera, Odonata) are stored directly in ethanol.
Small glass tubes ("microwells") with glycerin are used for small material, which are placed in a larger storage container. - Archiving of genital specimens
Isolated genitalia (especially for beetles and butterflies) are preserved in microvials with glycerin attached to a pin with the specimen or stored in a wet collection. - Research and museum collections
Containers are stored in shelves or storage cabinets, often by taxonomic group.
For larger specimens, hanging nets or gauze liners are used to prevent specimens from floating.
A constant temperature (15 - 20 °C) and low light exposure are maintained to prevent evaporation of the alcohol. - Short-term preservation of field samples
In the field, smaller jars or plastic vials are filled with alcohol for immediate fixation.
On return to the laboratory, the contents are filtered, labelled and transferred to standard storage containers.
The containerss are arranged in drawer filing systems or metal racks according to order, family, or location. They are placed in a single layer (not stacked) so that labels can be easily read and liquid levels checked. Each container has a unique inventory number linked to a database or collection book. Rubber pads and gloves are used for handling for both safety and container stability.
Descriptive box labels
Descriptive labels (sometimes also systematic, collection or header labels) are one of the important organizational elements of entomological boxes and collections. While determination labels identify individual specimens, descriptive labels are used to clearly organize entire groups of specimens within a box or drawer.
Descriptive labels in entomological boxes are used to:
- clearly label groups of specimens (order, family, genus, species, locality, etc.),
- facilitate orientation within the box or collection,
- separating taxonomic units (e.g. families or genera) within a single collection box,
- visual unification of the collection, especially for larger museum collections.
Descriptive labels are placed horizontally or vertically in the entomological box, depending on the practices of the collector or institution, usually in front of the first specimen of a given group or at the beginning of each taxonomic section. They are also often inserted at pin level between rows of specimens, or attached to the wall of the box. In modern collections, the system is usually arranged so that the main descriptive label (e.g., family name) is larger and placed at the top of the box, and subgroups (genera, species) are labeled with smaller labels in each row.
The size of the individual labels varies according to the level of description, e.g. for the main labels (order, family) sizes of about 30 - 50 mm × 10 - 15 mm are used, for genus and species labels then sizes of 15 - 30 mm × 5 - 10 mm and for e.g. local or project labels smaller sizes, e.g. 20 × 5 mm.
Systematic (taxonomic) arrangement is by far the most common way of arranging a collection, and is used in museums and research collections. Thus, the ordering is organized according to a taxonomic hierarchy: order ⟶ family ⟶ genus ⟶ species/subspecies. Within each taxonomic group (e.g. family), an alphabetical ordering of genera/species according to Latin names is then often used. This sorting method facilitates comparison, identification and access to taxonomic references and is used by all major national museums.
Another option is geographical (biogeographical) ordering, i.e. ordering by collection area (continent, country, region, locality). This type of ordering can be useful for faunal studies, biogeography or ecological purposes. The disadvantage is that taxonomic relationships of specimens are not in close proximity, making it more difficult to compare related taxa from different areas. In practice, geographic collections are often combined with systematic layering (i.e. within the geographic module the arrangement is taxonomic). Reference/regional collections often resort to this type of classification for easy access to local species.
Other ways of ordering specimens within a collection tend to be exceptional and are usually charged with the narrowly focused purpose of building that collection. They tend to be inappropriate for building an amateur collection, so we will not deal with them further.
Determination keys
Entomological identification keys are formalized systems that allow one to determine which species, genus, family or other taxonomic group of insects is involved based on observable characters.
Using a sequence of questions or choices that relate to external characters, the key will lead you to the correct order, family, genus, or species. This allows you to identify insects without laboratory methods, based on morphology alone.
Types of identification keys
- Dichotomous keys,
- The most common form,
- At each step, a choice is made between two opposing options,
- E.g. "Antennae longer than the body / Antennae shorter than the body",
- Polytomic keys,
- Offers more than two options at each step,
- They are less clear, but sometimes faster,
- Interactive and electronic keys,
- Web or mobile apps,
- The advantage is the ability to select characters in any order,
- E.g. determination using the "Lucid Key" or Czech portals like Biolib.cz.
How is the identification of insects done (step by step)?
- Sample preparation
Insects should be intact, preferably adults
- Inspection of the characters
Using a magnifying glass, binoculars or microscope, observe the morphology (e.g. shape and number of wings, antennae, legs, eyes, body (head, thorax, posterior), wing venation, labels, ...)
- Work with the identification key
- Start at the beginning and choose the option that corresponds to the insect character
- Follow the number to the next step
- If you get to the name of the taxon, you have identified the specimen
- It is advisable to check the identification in the literature or with photographs
Example of a dichotomous identification key (simplified version)
1a. Body soft, without wings → go to 2
1b. Body with wings or hard trusses → go to 3
2a. Has more than 3 pairs of legs → Class: Many-legged
2b. Has 3 pairs of legs → Class: Insects (Insecta)
3a. Has only one pair of wings → Order
3b. Has two pairs of wings → go to 4
4a. Forewings converted to hard scruff → Order: Coleoptera (beetles)
4b. All wings blanched → go to 5
5a. Hind wings larger than forewings → Order: Odonata (dragonflies)
5b. Wings of equal size → Order: Hymenoptera (blenocerans)
(This key is very simplified and is for illustration purposes only.)
The most common errors in identification
- Identifying immature stages (larvae, pupae) - other types of keys are used for this
- Neglecting small details (e.g. wing veining, shield shape)
- Working without good lighting or magnifier/microscope
- Use of outdated or inaccurate literature
- Failure to verify determinations from multiple sources
At the end, you should verify the taxon you have identified - the key often includes a description of the taxon, or a depiction (drawing or photograph) of important identifying features to check the accuracy of the identification.
Determination labels
Determination labels (sometimes also determination, identification or taxonomic labels) are one of the important elements of any entomological preparation.
The determination label is used to:
- mark the taxonomic identification of the specimen,
- a record of the person who identified the species (the determiner),
- documenting the date or revision of the determination,
- and, in some cases, to add information on the type or special status of the specimen (e.g., holotype, paratype, syntype).
These labels make it possible to accurately assign each specimen to a species and to the literature, to trace back to the author of the determination, and to verify the accuracy of the identification in later revisions or genetic studies. There are usually multiple labels one above the other on an entomological pin, and each has a specific purpose and sequence. The determination label is always placed below the locality label but above the supplementary (type or record) labels.
Basic parts of the determination label
| Data | Description | Example |
| Taxonomic name | Genus + species (or subspecies), including the name of the person who published the species description | Carabus (Procrustes) coriaceus coriaceus (Linaeus, 1758) |
| Identified by (determiner) | Name of the person who identified the species, with the abbreviation "det." (= determinavit) | det. J. Novák |
| Year of determination | Year (optional, but recommended for revision purposes) or date | det. J. Novák, 2024 or det. Novák 12.VI.2024 |
Other possible additions:
- rev. (= revisus) - when the determination is reviewed by another expert,
- conf. (= confirmavit) - confirmation of the correctness of the determination,
- cf. or aff. - denotes uncertainty of determination (e.g. cf. Carabus violaceus = "probably this species").
Recommended dimensions are commonly given as 10 × 5 mm to 20 × 8 mm, depending on the size of the insect. I recommend using the same dimensions as for the locality label.
Some institutions use a color-coded label that might look something like this:
| Colour | Meaning |
| White | common specimen, standard designation |
| Red | type specimen (holotype, paratype) |
| Yellow | revision or confirmation marking |
| Green | specimen from a research project |
| Blue | genetically processed specimen (DNA sample) |
Determination labels are closely linked to the records:
- Each determination (including revisions) is recorded in the collection database,
- The name of the determiner, the date and the literature or method used are given,
- When the name is later changed, the original label is retained, and a new label is added (not replaced).
- This allows the history of the specimen identification to be tracked.
Entomological boxes
Entomological (sometimes called collection) boxes are designed for long-term preservation of entomological specimens in scientific, museum, educational, and amateur collections. These boxes represent the highest level of preservation and archiving of specimens and form the basis of any professional entomological collection.
Entomological boxes are designed for:
- long-term preservation of entomological specimens (pinned or glued to labels),
- protection against mechanical damage, moisture and pests,
- a stable physical and chemical environment for preserved specimens,
- systematic arrangement of the collection according to taxonomic groups,
- easy access for scientific work, identification or inventory.
They are therefore a permanent storage facility - specimens are permanently classified in these boxes after processing and identification. The entomological box is designed to be strong, sealable, dust-tight and chemically neutral.
A classic entomological box usually consists of
- The body (frame)
Constructed of high quality hardwood (beech, maple, walnut, birch) or multi-ply plywood. The wood must be dry and resin-free, the surface usually varnished or waxed. The height of the frame is usually about 5 - 6 cm to provide sufficient space for impaled specimens. - Bottom (entomological plate)
Inserted into the frame and forms the main working surface for specimens. The material is nowadays exclusively entomological foam (Ethafoam, Plastazote, Poreten, ...). The bottom must be flat, flexible and non-sticky so that the pins can be easily inserted and removed again. - Lid
May be glazed (glass or plexiglass) or solid. The transparent lid allows the collection to be inspected without opening. It is fixed with hinges or a snap closure, and is sometimes removable in its entirety. The edges of the lid are usually fitted with a seal (felt, silicone tape, rubber insert) to keep out dust and small pests. - Internal arrangement
The internal space is usually uniform, for free distribution of the specimens according to the systematics. In some cases, there are low separating strips inside for clear division of families or genera. The boxes are usually numbered and labelled on the front or lid. - Markings and accessories
A label with the group name (e.g. 'Coleoptera - Carabidae') is usually on the front. Inside may be repellents (e.g. thymol, lavender sachets, or modern formaldehyde-free alternatives). A silica gel or moisture indicator is inserted to prevent moulding of the preparations and corrosion of the pins.
The most common standardised dimensions (length x width) of entomological boxes are 23 x 30 cm, 30 x 40 cm and 40 x 50 cm. The heights of the boxes tend to be 5.4 cm (space saving, sufficient for standard entomological pins and labels), 6 cm (more versatile, e.g. common in the National Museum), or 8 cm (special cases for larger specimens).
After identification and labelling, specimens are placed in entomological boxes according to the taxonomic system. Each specimen has a label with information about the collection, locality, date and collector. The boxes are placed in collection cabinets or shelves - usually in groups of tens to hundreds.
UNIT SYSTEM
Entomological boxes called UNIT SYSTEM are also used in the Czech Republic. UNIT SYSTEM boxes are special types of boxes designed for systematic and modular storage of insect collections. Their main idea is compatibility and the possibility of inserting smaller, separately removable units (cardboard boxes, pads or plastic boxes) into the basic box.
Characteristics of the UNIT SYSTEM:
- Modularity and flexibility
The base box is designed so that smaller boxes/boxes can be freely arranged within it, allowing easy handling, rearranging and operational work with the material in the box without having to carry the entire collection. - Lack of solid bottom filling
UNIT SYSTEM boxes are sold without bottom filling (e.g. without polystyrene or plastazote), as this filling is found in the smaller insertion units. - Material
Both solid wood (e.g. alder, pine, stained mahogany) boxes and coated (e.g. canvas, cardboard/wood with coating) variants are available. - Lid
Variants with full lid or glass lid are available. - Marking
A distinction is sometimes made between UNIT SYSTEM - CLASSIC and UNIT SYSTEM - PLAST, depending on the type of smaller units inserted with which the box is compatible.
Typical dimensions (external, examples) of each UNIT SYSTEM:
UNIT SYSTEM - CLASSIC:
- 30 x 40 x 6 cm (all-wood)
- 40 x 50 x 6 cm (all-wood)
- 30 x 40 x 5.4 cm (glued)
- 40 x 50 x 5.4 cm (glued)
UNIT SYSTEM - PLASTIC:
- 31.5 x 38 x 6 cm (all wood)
- 31,5 x 38 x 5,4 cm (glued)
Insecticide cups
Insecticide cups in entomology boxes (or also "nitrobenzene cups" or "disinfestation cups") are used in entomology to protect collections against pests (e.g., weevils). These are usually small glass flasks or cups with a lid and a stainless steel tip that is stuck into the bottom of the entomological box containing the prepared insects. A chemical (nitrobenzene used to be used, but nowadays other less toxic substances are more common) with an insecticidal effect is inserted or dripped into the cup, which slowly evaporates and maintains an environment in the box that protects the insects from destruction.
They can also be small containers with cotton wool or felt soaked in the chemical. They are either open or closed with a lid and holes (to allow vapour to escape) but prevent direct contact of the specimens with the chemical. They are usually fixed in the corner of a drawer or entomological box.
Key features and purpose:
- Protection of collections - prevent pest insects from attacking and destroying collections.
- Chemical Application: Used to safely and gradually release a protective substance into the enclosed space of the box.
- Design: They are usually glass, with a stainless steel tip for easy insertion into the bottom of the box (which is usually made of plastazote or other suitable filler).
- Sealed or with a lid - the lid ensures controlled and slow evaporation, prolongs the effectiveness and prevents rapid evaporation or contact with insects.
These cups are a common piece of equipment for any serious insect collector, although these days they tend to be replaced by other, more gentle collection protection methods.
Labeling pins
Labeling pins are used in entomology exclusively for attaching and fixing various types of descriptive labels inside entomological boxes or museum drawers. They are very short and thin pins (often around 10 mm in length) that are different from standard entomological pins (which are used to impale the insect itself).
The main purposes of labelling pins
- Attaching group labels
These are used to attach paper or plastic labels with the names of higher taxa (order, family, genus, species) directly to the bottom of the entomological box. These labels are not pinned directly through the centre (to prevent the paper from curling), but with two pins on each side, which ensures stability and the possibility of replacement without damaging the substrate. They are used, for example, for labels that separate individual taxonomic blocks. - Space demarcation and orientation
Labelling pins can also be used to separate sections (e.g. between families or genera) by attaching tapes or tabs. They are also sometimes used to mark blank spaces for future specimens or to complete a series. - Marking type material or special groups
To mark type specimens (holotype, paratype) or other significant pieces, a small coloured label attached with a labelling pin may be stuck close to the specimen. The colours or shapes of such labels are used for easy visual orientation. - Temporary labels for sorting
When processing collections or revisions, labeling pins are used for temporary labels that can be easily removed or moved without handling specimens.
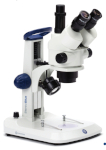
Magnifier / Microscope
Magnifying loupes/microscopes that provide sufficient magnification but also a comfortable working space (working distance) and good lighting are important for insect identification.
When choosing a magnifier, similar requirements apply:
- basic optical magnification (choose magnifiers with magnification up to 20×)
- magnification selection method (I recommend magnifiers that can be reconfigured for different magnifications)
- quality of optics (also when choosing a magnifier, look at the quality of the optics; lower quality means distortion, chromatic defects)
Pocket (retractable) magnifiers typically have magnifications of 10x - 20x and are Ideal for detailed viewing of smaller structures (antennae, legs, wing veins) and for field work. They are compact but require good lighting.
Head (spectacle) magnifiers have a wider magnification range of 1.5x to 25x and are invaluable for preparation and handling of small insects. They allow you to keep your hands free. They usually have integrated LED lighting.
Handheld magnifiers (with handle) have magnifications of 3x to 10x and are used for quick inspection of larger specimens or checking locality labels. They often come with LED lighting, which is a great advantage.
When choosing a microscope, I recommend looking at the following parameters:
- overall rigidity of the construction (holder, stand or arm to ensure accuracy and the possibility of gentle movement)
- basic optical magnification (must be able to see fine features such as striations, projections or bristles; choose stereo microscopes with magnification of at least ≧ 90×)
- the choice of magnification (fixed vs. zoom: each has its advantages and disadvantages, I prefer zoom for convenience with microscopes)
- the minimum distance of the objective lens from the object to be observed (this determines the possible working space; when using the microscope also for preparation it is at least 80 mm)
- illumination (important when observing details; I recommend LED lighting from above (reflective) or ring lighting around the objective)
- options for accessories (eyepieces, objectives, filters, eyepieces)
- quality of optics (lower quality means distortion, chromatic defects)
- photographic recording possibilities (e.g. third tube)
Binocular stereoscopic microscopes (or binocular magnifiers) are the most important and most used tool for serious insect determination. It provides a stereoscopic (3D) image and allows observation of details in a relatively large field of view.
For extremely small insects and genitalia, a conventional biological microscope is used. This is used to identify very small species (e.g. some parasitic whiteflies, small beetles, thrips) or to study internal structures. Its magnification is usually 100x to 400x. It requires special microscope slides (embedded in resin, e.g. Canadian balsam or Euparal) or temporary slides in liquid.
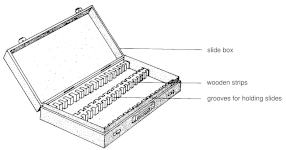
Microscope slide boxes
Microscope specimen boxes (called microscope trays or microscope boxes) are an important part of many entomological collections because they protect permanent microscope specimens (e.g., insect body parts, genitalia, larvae, parasites, fragments, etc.) from damage, moisture, and contamination.
These boxes are used to:
- safe storage of microscopic slides on glass,
- protection from dust, light, moisture and mechanical damage,
- systematically arranging slides by species, location or date,
- easy handling and inventory of the collection material,
- transfer of slides between the laboratory, the depository and the microscope.
They are used in museum, university, research, and amateur entomological collections, where they are complementary to collections of macroscopic (pinned, glued) specimens.
They usually consist of
- The body (body of the box)
Most often made of solid wood, plywood, hard cardboard, or plastic polymer (ABS, PP). Emphasis is placed on strength, dimensional stability, and chemical neutrality (must not release fumes that would affect the adhesives or medium on the slide). The surface is usually smooth, varnished, waxed, or plastic for easy cleaning.
Typical dimensions:
- small boxes: approx. 15 × 10 × 3 cm (25 - 50 slides),
- medium boxes: approx. 20 × 15 × 4 cm (100 - 150 slides),
- large trays: approx. 30 × 20 × 5 cm (up to 300 slides). - Internal layout
The internal space is divided into rows or compartments into which microscope slides (standard 75 × 25 mm) are inserted.
The compartments are:
- lined with felt, paper or foam,
- fitted with separating rails of plastic or wood,
- sometimes marked with numerical indices (e.g. 1 to 50, 1 to 100).
Some modern plastic boxes have insertion strips with flexible cut-outs that hold the slides firmly but do not damage the glass. - Lid
Usually solid, made of the same material as the body of the box, or glazed (Plexiglas) for inspection of the contents. It is secured by hinges, latches or clips. The inside of the lid may have an inventory plate - for numbering the slides and recording data (species, locality, sex, date, etc.). The closure should be tight to prevent the entry of dust and insects. - Labelling and accessories
A label with the group name or inventory number is usually on the front. An internal chart allows each specimen to be assigned a number (identical to that in the database or collection catalogue). Some modern boxes also contain a QR code or electronic chip for digitised records. Moisture absorbers (e.g. silica gel) and pest repellents are also included.
Types of microscope boxes
| Type | Material | Capacity | Application |
|---|---|---|---|
| Wooden classic box | Hardwood, felt bottom | 50 - 100 slides | Traditional museum collections |
| Cardboard (archival) | Acid-resistant cardboard | 25 - 100 | Short-term or educational storage |
| Plastic laboratory box | Polypropylene / ABS | 50 - 200 | Modern laboratory, moisture resistant |
| Drawer microscope archive | Metal or plastic frame with drawers | 500 - 2 000 | Large collections, museums |
| Mini box (portable) | Plastic or plywood | 10 - 25 | Transport of slides to the microscope |
Quarantine box
A quarantine box is nothing more than a special box designed to carry out the quarantine process when a foreign specimen is acquired (e.g. from an exchange). This process is designed to secure the acquired specimen so that it cannot potentially infect our collection with pests. This is because newly acquired specimens may contain eggs, larvae or adults of pests (most commonly disturbers, leatherjackets, moths or weevils) that would spread quickly.
Here is a best practice for protecting your collection when acquiring foreign specimens:
- Quarantine
- Never add newly acquired specimens directly to an existing collection.
- Isolation: place them in a separate, well-sealed box or container (quarantine box) and store them away from the main collection (ideally in another room as well). Quarantine should last at least 4 - 6 weeks.
- Inspection: during quarantine, check the box regularly for signs of pests (fine dust under specimens, larvae, adults, damage).
- Treatments (disinfestation)
All new specimens should undergo a preventive treatment that reliably eliminates any developmental stages of pests- A. Freezing (the most common and safest method)
- The most effective and most commonly used method, which does not damage dry specimens (if they are dry enough and do not get stuck):
- Packing: place specimens in a tightly sealed bag (e.g. zip-lock bag, plastic box or direct quarantine box), ideally with a small amount of cellulose cotton wool or paper towel to keep them from moving.
- Freezing: place them in the freezer at -18˚C or below for a minimum of 48 to 72 hours (2 - 3 days). A longer period (e.g., a week) is even safer.
- Acclimatisation: After removing from the freezer, allow the bag/box to slowly acclimatise to room temperature for a few hours (approx. 12 - 24 h) without opening it. This will prevent condensation of moisture inside which could damage the preparation or cause rust on the pins.
- Control: After acclimatization, perform a thorough visual inspection.
- B. Chemical treatment
- Traditionally, chemical agents (e.g., naphthalene, thymol, paradichlorobenzene) have been used for disinfestation and placed in boxes as a repellent or exterminating agent.
- Products: some entomologists use special insecticidal tapes or products designed to protect collections that are inserted into boxes (e.g. Dermestes Stop or similar products). However, these often serve more as prevention for the permanent collection and may not be effective enough to immediately eradicate the invasion in new material.
- Fumigation: larger collections or heavily infested material are sometimes treated by fumigation (gassing) in museum practice, but this requires special equipment and is a rather radical and dangerous solution for a domestic collection.
- A. Freezing (the most common and safest method)
- Incorporation into the collection
Only after the specimens have undergone quarantine and preventive frost treatment (or other proven method) and have been thoroughly inspected for signs of pests, can you place them in your permanent collection.
Storage box
Storage boxes for the short-term storage of specimens (also called working, temporary or sorting boxes) serve as temporary storage for specimens prior to their final classification in the main collection. They are used after the slides have dried out before they are sorted into the collection.
Storage boxes are intended for:
- storing freshly prepared slides that have not yet been identified, checked or classified,
- temporary storage of specimens recovered from the field or exchanges,
- temporary sorting by locality, collector or date of collection,
- protection of specimens between preparation and archiving.
They are commonly used in research laboratories, museums, universities and amateur collections. In amateur settings, they are often replaced by older entomological boxes.
A storage box usually consists of:
- The body (which is usually made of lightweight wood, plywood, or rigid cardboard; it does not need to be as sturdy as transport or entomology boxes; light weight, easy stackability, and inexpensive manufacture are important),
- Bottoms and fillers (the interior is lined with foam (Ethafoam, Plastazote, polystyrene, ...); the material must allow re-pinning and removal of slides without damaging the pins; it is assumed that the filling changes frequently according to wear and tear),
- Lid (may be removable or hinged, often glazed or made of Plexiglas to allow inspection of the state of the slides without opening; closure is usually simple - e.g. rubber band, latch, clip).
- Labelling (on the lid or side there is space for temporary labels - e.g. collection number, location, collector's name or date; for larger collections colour codes or stickers are also used according to the stage of processing (e.g. designated/undesignated/inscribed)).
Test tube
Epruvettes (test tubes) are cylindrical, closed-bottomed, open-necked containers designed to hold biological material in a liquid or dry environment.
In entomology, they are mainly used for(e):
- collection, transport and storage of insects (live and dead),
- preservation of specimens in liquids,
- preservation of larvae, pupae, parasites or genital specimens,
- preparation of microscopic slides,
- chemical or morphological experiments (e.g. pigmentation tests, pH measurements, etc.).
Glass or plastic tubes are used. Glass test tubes are made of classical laboratory glass (borosilicate - e.g. Pyrex, Duran) and are resistant to chemicals, alcohol, ether and boiling. They are mainly used in the laboratory (decolourisation, cleaning and maceration of insect parts (e.g. genitalia, wings, larval parts), preservation of microscopic parts before mounting in embalming, temporary storage in KOH or chloral hydrate solution) and for permanent preservation of specimens (for long-term preservation, specimens are placed in tubes with preservative liquid).
Plastic tubes are made of polypropylene (PP) or polyethylene (PE) and are therefore lightweight and unbreakable. They are mainly used for field collection and transport. They are used, for example, for the rapid collection of smaller specimens. Small tubes (< 5 ml) are often used for genital specimens, stored e.g. in glycerol or microspheres attached to the specimen pin (see e.g. Fernandes André Silva, Guedes Joab Cardoso, Krolow Tiago Kutter: A step-by-step guide for manufacturing a reliable and low-cost entomological dissection microvial for pinned specimens; Contributions to Entomology, 75 (2), (2025), p. 263-268).
Transport boxes
Transport boxes for entomological specimens must be designed to ensure safe, stable and dry transport of sensitive insect collections. They are used for the temporary movement of specimens, e.g. when exchanging preparation material at exchanges.
The transport boxes protect the slides from:
- mechanical damage (shocks, impacts, friction between pins),
- moisture and temperature fluctuations,
- infestation by pests (e.g. dermestids, moulds).
The box usually consists of:
- a rigid outer box - wood, plywood, plastic or metal frame,
- inner filling - EVA, polystyrene, Ethafoam, Plastazote, Poreten,
- lid with seal - for dust-tight and partially airtight closure (solid or transparent).